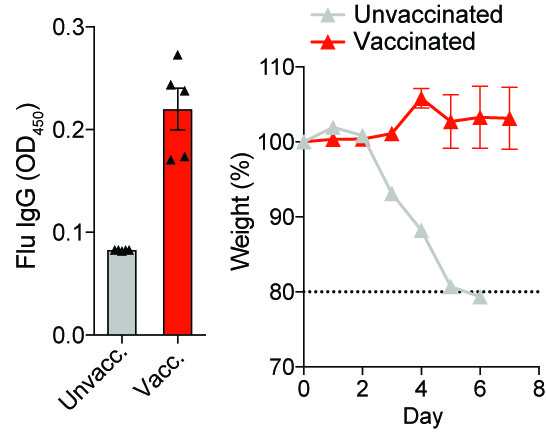
Antibody Regulation During Influenza and SARS-CoV-2 (COVID19) Vaccination and Infection. Production of high affinity antibodies is the basis of protection elicited by most vaccines. However, most vaccines are empirically developed. Antibody responses are controlled by follicular T cell populations in germinal centers. One focus of the lab is to understand how specific follicular T cell populations can control vaccine responses. The seasonal influenza vaccine does not provide complete protection, even in healthy young adults. We use clinically relevant influenza vaccines to understand how follicular T cell populations can control vaccine-elicited protection from influenza infection. In addition, we are studying how these same populations can be mobilized during viral infection to enhance immunity. We are also studying how follicular T cell populations can be targeted to induce augmented antibody responses in the context of SARS-CoV-2 (COVID-19) vaccines and infection. These studies will help determine new strategies to enhance immunity during vaccination and infection.
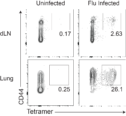
CD8 Memory to Intranasal Infections. CD8 T cells are essential for the elimination of viruses that infect the lung mucosa, such as Influenza and SARS-CoV-2. Memory CD8 T cells are particularly important upon secondary exposure to viruses. However, the transcriptional machinery that facilitates memory CD8 T cell formation and function is still not completely known. We use clinically relevant influenza infection models to study the cellular and molecular basis for CD8 memory. These studies will help identify ways to enhance the immune system to fight infections of the lung.
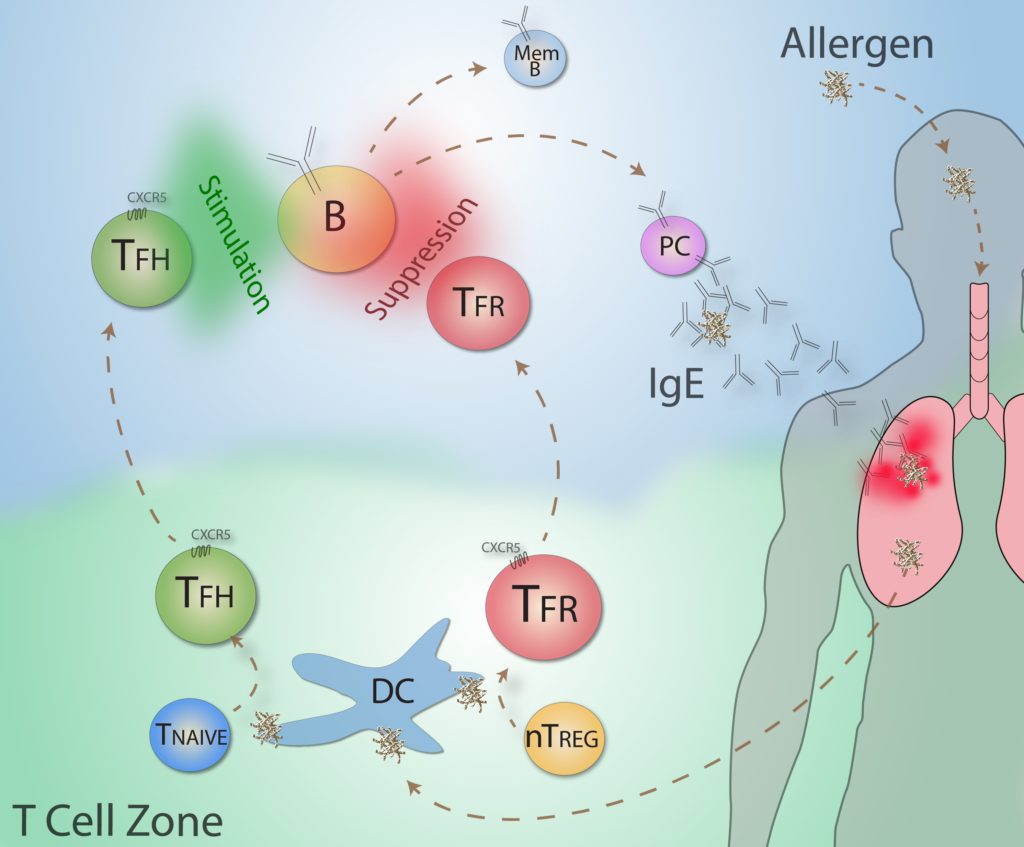
Antibody Regulation in Allergic Airway Disease. The incidence of allergies is increasing world-wide. Allergies are induced and exacerbated by pathogenic IgE antibodies which cause degranulation of mast cells leading to inflammation. We have shown that a specific subset of Tfh cells called Tfh13 cells are specialized at inducing IgE responses to allergens. Furthermore, we have shown that Tfr cells regulate IL-13 production by Tfh13 cells as well as allergic airway disease. Although specific Tfh cell subsets and Tfr cells can control allergies, the nature of this regulation is currently unknown. We are using novel in vivo mouse models to study how follicular T cell subsets can control allergic airway disease. By understanding how allergies are regulated, new therapeutics can be developed to inhibit allergies and asthma.
Regulation of Antibody Mediated Rejection During Transplantation. Currently, there are ~100,000 patients on the kidney transplant waiting list. ~5% of patients die each year while waiting for a matched kidney, due to a lack of donated organs. Even when patients are lucky enough to undergo transplantation, graft rejection can occur over time. Long term graft rejection is due in part to antibody mediated rejection (AbMR). Very little is known how allospecific antibodies are controlled during transplantation. A focus of the lab is to understand how the immune system controls allo-specific antibodies and how these antibodies can lead to AbMR. We use novel mouse models in combination with clinically relevant solid organ transplantation to determine how follicular T cell subsets promote and inhibit AbMR. By understanding how the immune system regulates allospecific antibodies new therapeutics can be developed to limit AbMR in patients.
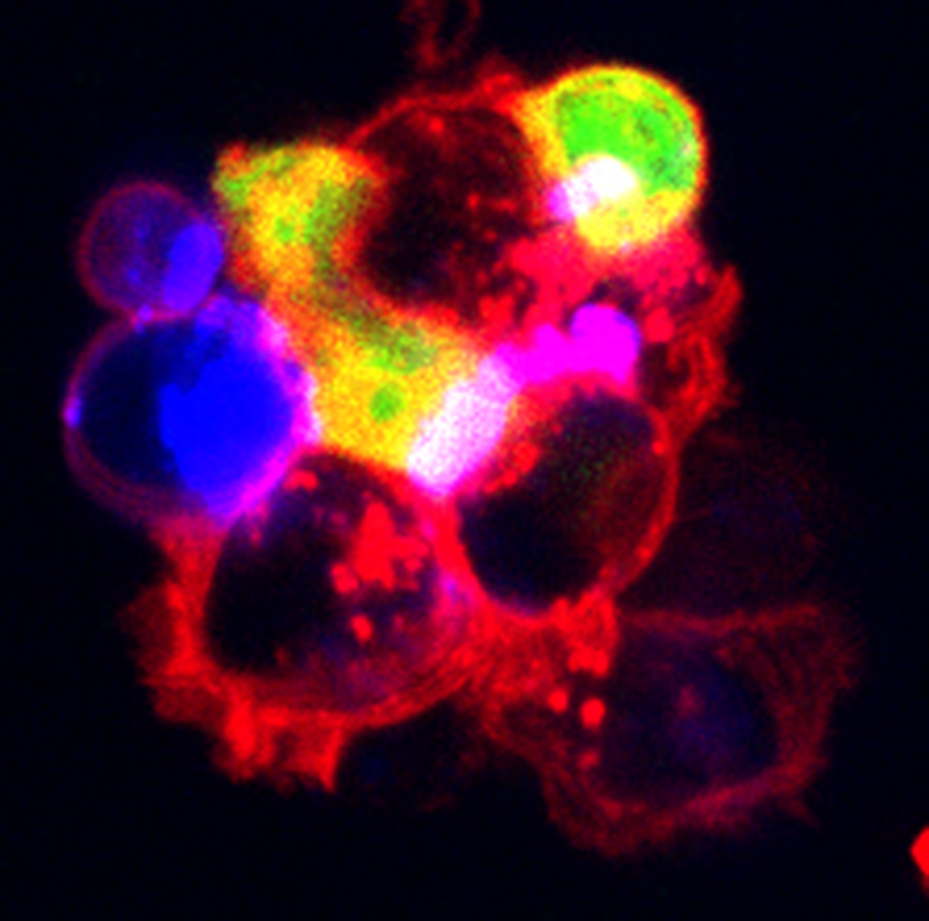
Germinal Center Communication. In the germinal center reaction, Tfh and B cells interact in a dynamic and intimate process called linked recognition. This interaction instructs effector potential of both cell types leading to Tfh cell cytokine production and B cell somatic hypermutation. Tfr cells inhibit these responses. However, the mechanisms facilitating cellular communication between Tfh, Tfr and B cells is still unknown. Therefore, a focus of the lab is to understand mechanistically how Tfh cells interact with B cells, and how Tfr cells can alter this cellular communication. By understanding the nature of this communication, therapies can be developed that enhance vaccine responses or limit systemic autoimmunity.
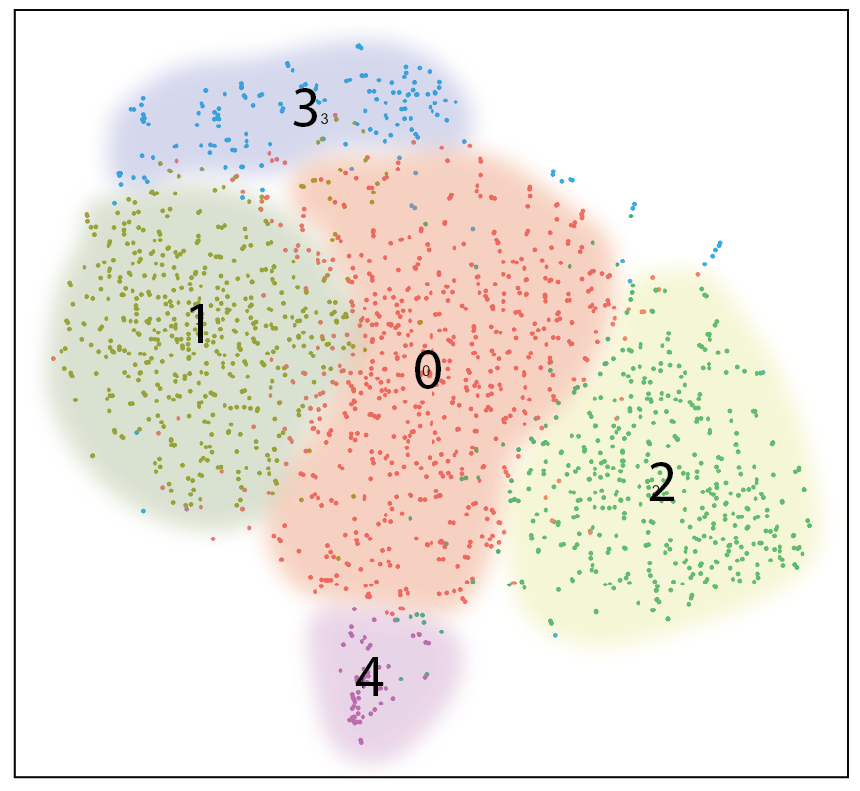
Transcriptional Regulation of Tfh and Tfr Cell Identity. Tfh cells stimulate whereas Tfr cells inhibit B cell responses, yet they share substantial overlap in transcriptional programming. A focus in the lab is understanding the transcriptional programs facilitating functionality of follicular T cell subsets. We recently shown that the Tfr program combines features of a Treg program and a Tfh program to facilitate suppression. We are using a combination of novel reporter mice, human samples, bulk and single cell RNAseq transcriptional analysis to uncover how follicular T cell subsets are wired to perform their functions. Uncovering these pathways will allow targeted approaches to enhance specific follicular T cell subsets to promote or inhibit antibody responses.
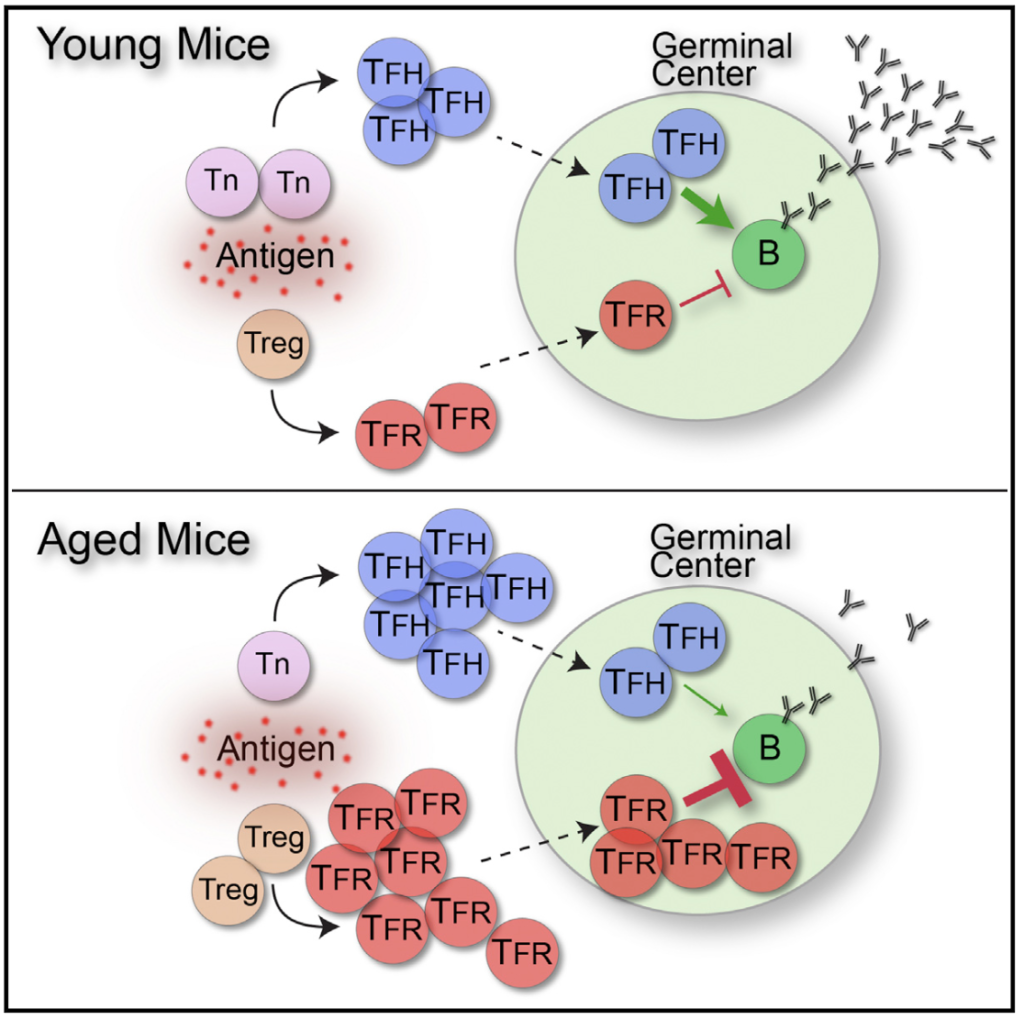
Aging of the Immune System. Vaccination has eradicated many harmful pathogens. However, in some cases vaccination does not provide complete protection. For instance, the elderly mount ineffective vaccine responses. This is due in part to age-related changes in the immune system such as thymic involution, expansion of memory cells, and alterations in cytokines. We have previously shown that in aged mice Tfh cells are dysfunctional and Tfr cells greatly expand, leading to suppression of antibody responses. Currently, a focus of our lab is to understand how aging alters germinal center populations and antibody responses to relevant vaccines. For this we use in vitro and in vivo assays and Flu vaccination in pre-clinical models of aging. By understanding age-related changes in the immune system, new therapeutics can be developed that enhance vaccines in the elderly as well as general population.
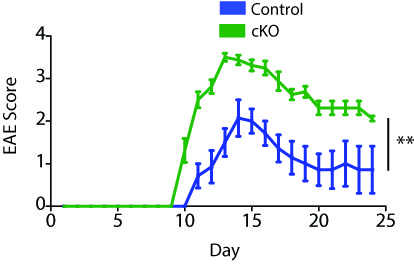
Control of Autoimmunity By Follicular T Cells. B cells play an important role in controlling both tissue-specific and systemic autoimmunity such as Multiple Sclerosis and Lupus, respectively. However, it is likely that B cells have distinct roles in the progression of autoimmune diseases. A focus of the lab is in trying to understand how B cell regulation controls the initiation, progression and resolving of autoimmune diseases such as Lupus and multiple sclerosis. For this, we are using novel genetic strategies in conjunction with experimental autoimmune encephalomyelitis (EAE) or lupus predisposition to understand how B cells control autoimmune disease. These studies will help identify new strategies to inhibit autoimmune disease pathology.